Risk-based data science supports the proactive identification, assessment, monitoring, and mitigation of risks in clinical trials using Quality by Design (QbD) principle.
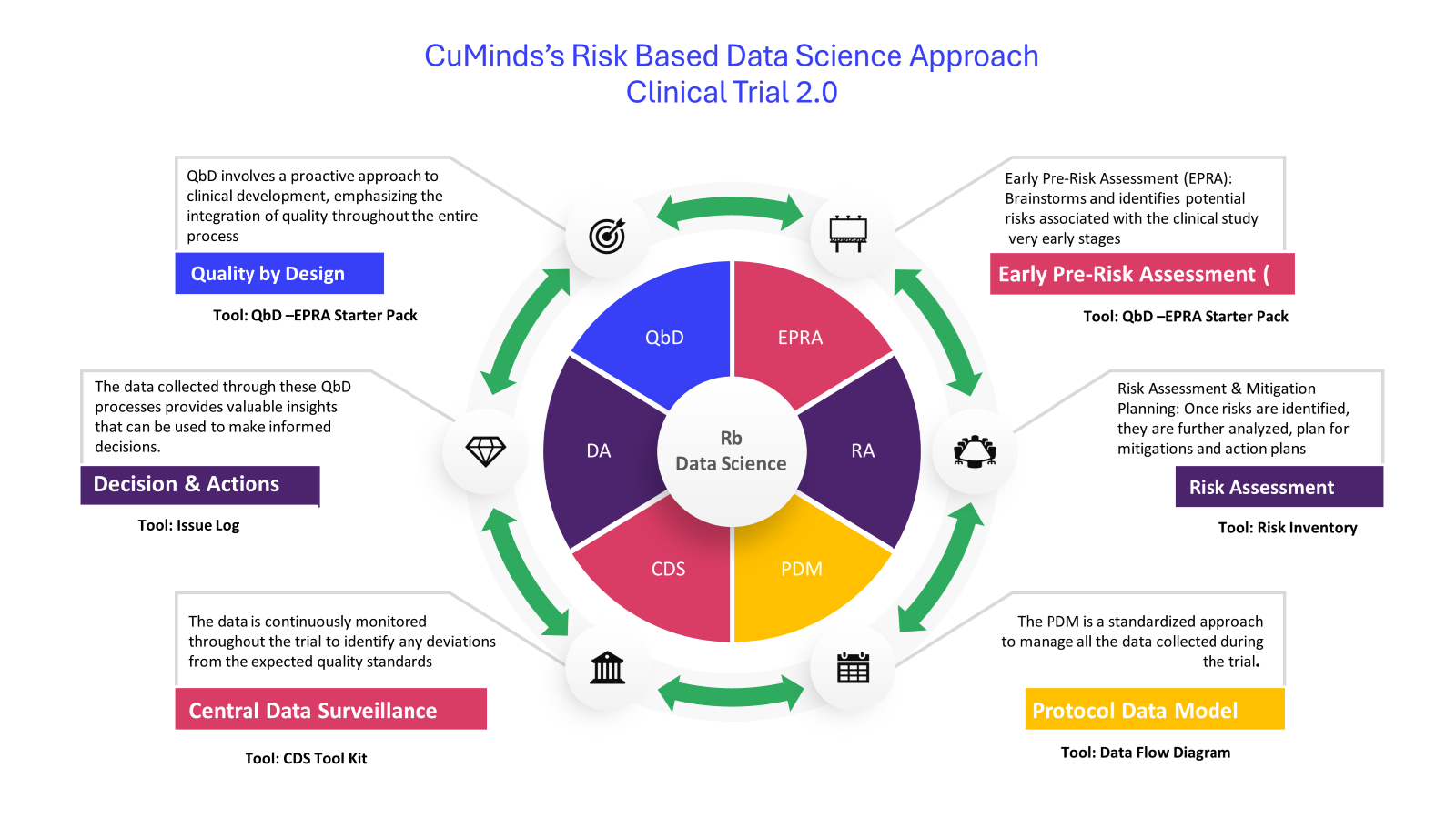
Risk-based data science supports the proactive identification, assessment, monitoring, and mitigation of risks in clinical trials using Quality by Design (QbD) principle.

| Approach | Technique | Tool/Aid |
|---|---|---|
| Quality By Design (QbD) |
|
|
| Risk Based Data Science |
|
|
By following the QbD approach, you gain greater control over your clinical trials and ensure safety and efficiency. Here are some of the key benefits:
In essence, Risk-Based Data Science powered by QbD is a smarter way to conduct clinical trials. It leverages data to proactively manage risk and ensure the quality of the data you collect. This leads to more efficient trials, better quality data, and, ultimately, the development of safer and more effective products.


Deep Expertise in Clinical Research:

Stay Long (more than 3 Months)
Long-term Engagement: Delve profoundly into your projects with our team accompanying you for an extended span, ensuring sustained success and growth.
Stay short (1 to 3 Months)
Short-term Engagement: Our short-term Engagement is meticulously designed to tackle specific challenges efficiently and effectively for urgent and precise problem-solving. This tailored approach ensures that your immediate needs are met precisely and quickly.
Stay Quick (Less than a Month)
Rapid response Engagement: When time sensitivity is paramount, our rapid response team promptly initiates action, delivering solutions quickly and accurately


Chief Data Scientist
With over 16 years of experience in the clinical research industry, Mukesh has worked with companies that provide next-generation technology solutions for RBQM, Data Management, and Data Science. Mukesh is a trained pharmacologist with expertise in various therapeutic areas and phases of clinical research.
As the Product Owner and SME for Central Monitoring and Clinical Technologies, Mukesh has extensive experience in the implementation and optimization of RBQM tools and processes, such as Central Statistical Monitoring, Quality Tolerance Limits, and Risk Indicators. His mission is to drive innovation and excellence in clinical research with data and technology, and to collaborate with internal and external stakeholders to ensure quality, compliance, and efficiency.
Talk to Expert